When Canada made history by legalizing cannabis nationwide for recreational use, something was noticeably missing: edibles and extracts.
And now, just over two months into the federal legalization of adult-use cannabis in Canada, the Liberal government has released proposed regulations for edibles and extracts.
As of October 17, 2018, Canadians of legal age have been able to purchase dried and fresh flower, and ingestible oil, leaving a large portion of cannabis products on the market unregulated. But as of Dec. 20, 2018, legal cannabis-infused edibles, extracts and topicals will be made available by Oct. 17, 2019, the anniversary of national legalization, according to the newly proposed rules.
In a news release, Health Canada issued a mea culpa of sorts, stating:
The old approach to cannabis did not work. It let criminals and organized crime profit, while failing to keep cannabis out of the hands of Canadian youth. In many cases, it has been easier for our kids to buy cannabis than cigarettes.
The complete list of proposed regulations will be published in the Canadian Gazette on Dec. 22, 2018. The public may request a copy of the regulations pre-publication by emailing cannabis@canada.ca directly.
Weedmaps News put together the key takeaways from the freshly proposed regulations in anticipation of the public consultations and finalized rules.
1. Edibles, Concentrates, and Topicals Will Have THC Limits
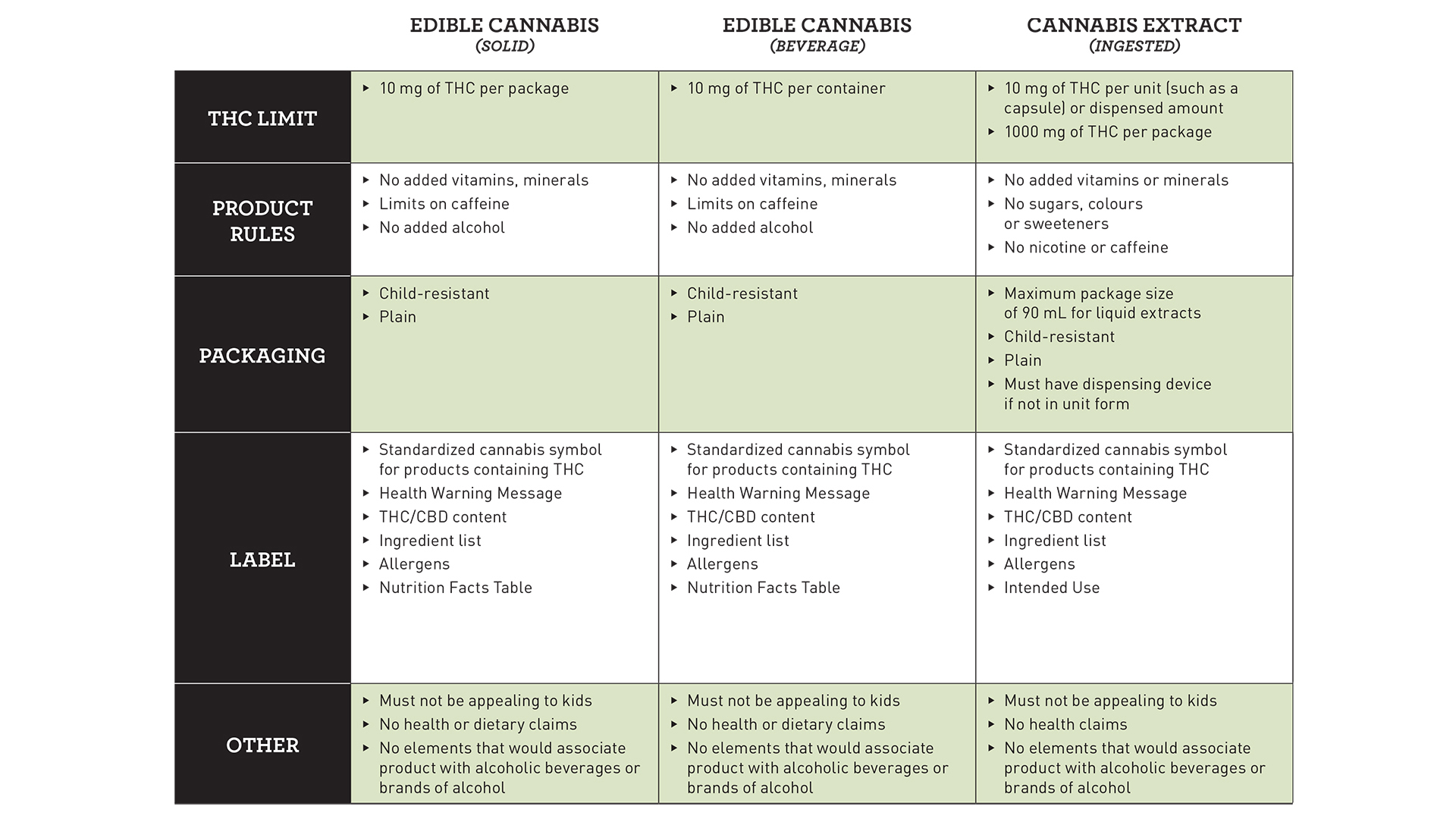
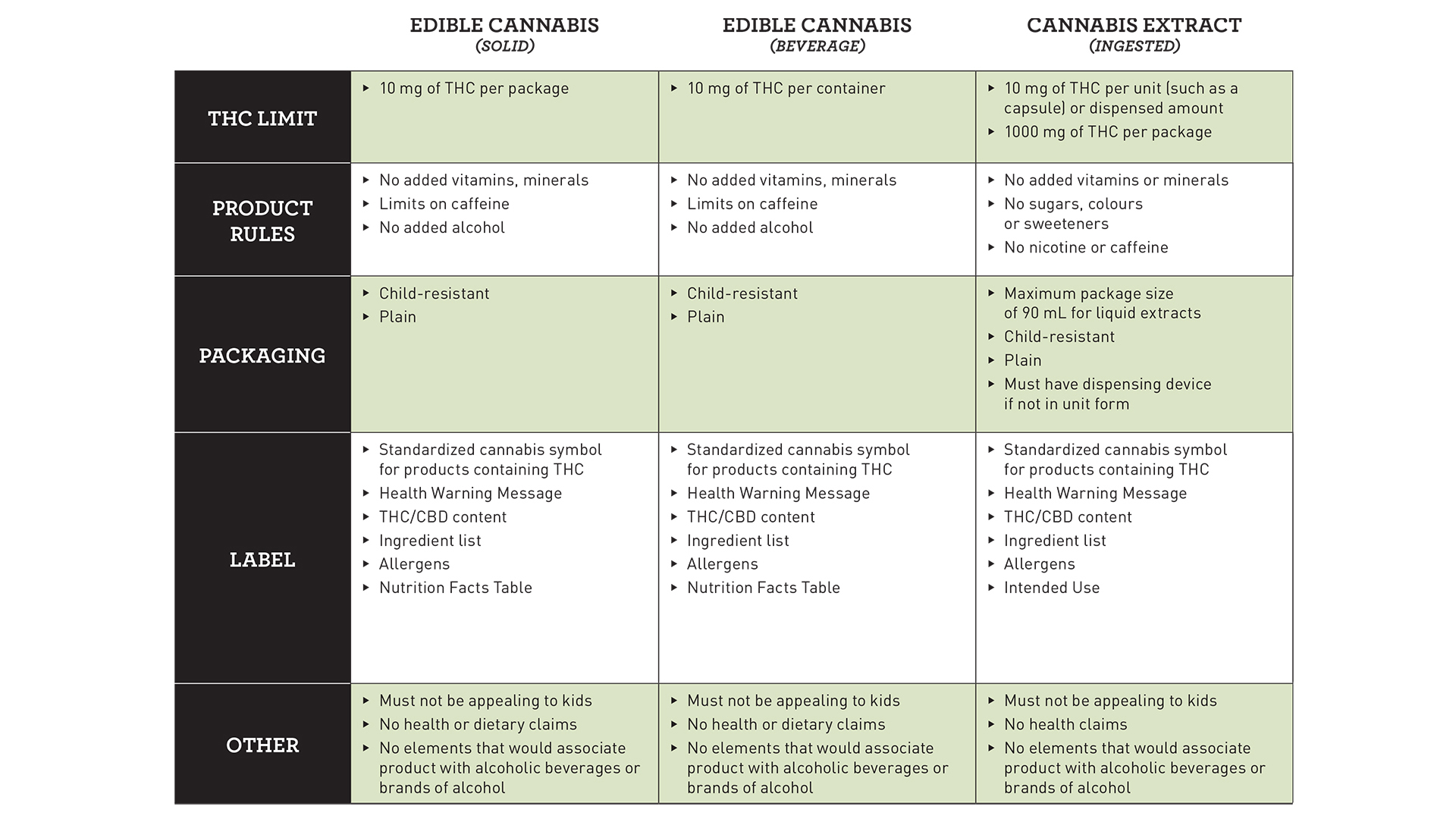
Solid edibles and beverages have a proposed limit on THC, the main intoxicating compound in cannabis, of 10 milligrams per package, while capsules have a proposed limit of 10 milligrams per dose and 1,000 milligrams per package.
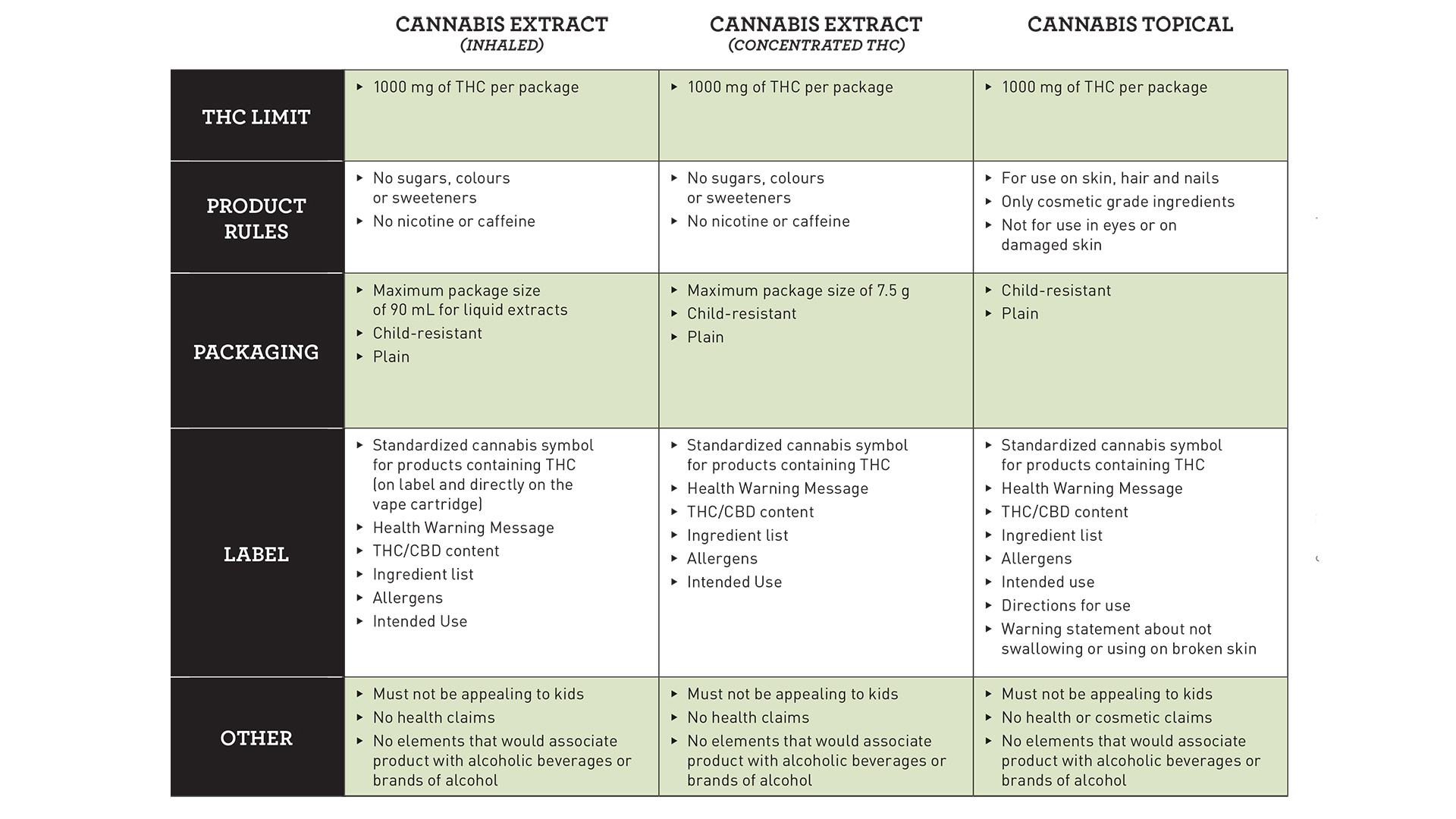
Concentrates and topicals both have a proposed THC limit of 1,000 milligrams per package.
2. Some Ingredients Might Be Restricted
There are various proposed restrictions on edibles including no added vitamins, minerals, alcohol, and limits on caffeine.
Extracts, which are subdivided into ingested, inhaled, and concentrated THC, could see restrictions on the use of sugars, vitamins, minerals, colours, sweeteners, caffeine, and nicotine, depending on their category.
Source: Health Canada
3. Packaging and Labeling is Still a Priority
As with all legal cannabis products in Canada, packaging must be plain and child-proof. In addition to the standardized cannabis symbol for products containing THC, health warning message, and THC and CBD content, labelling is proposed to include an ingredient list, allergens, and a nutrition facts table.
Part of Health Canada’s stated public safety approach to cannabis is to ensure that products would not be appealing to children. Packaging and marketing restrictions are one element of that, although it has yet to be seen how that will be regulated when it comes to cannabis-infused foods and beverages.
4. No Health Claims
Furthermore, no health claims can be made, which is consistent with the current regulations for legal cannabis, and no association can be made with alcoholic beverages or brands of alcohol.
Source: Health Canada
5. The Public Has 60 Days to Comment on New Regulations
In a statement, Minister of Border Security and Organized Crime Reduction Bill Blair said, “The Government of Canada’s top priority is the health and safety of Canadians. By establishing a strict regulatory framework for these new cannabis products, we are keeping profits away from criminals and organized crime. I encourage all interested Canadians to share their views on the proposed regulations.”
The draft regulations will be open for a public consultation period until Feb. 20, 2019. Comments can be submitted online or by mail.
Jessica Brown
Jessica Brown is a cannabis enthusiast with over 20 years of knowledge and appreciation that continues to grow. Through her background in psychology and creative writing, Jessica explores the worlds of cannabis, sex, and mental health. When she’s not in front of her computer or food, you can easily find her hiking up a mountain or snowboarding down one.